Basic Financial Statements
Select a chapter above and press 'Show Content'. Click a video topic below to view.
- Accounting Principles
- Separate Entity and Going Concern Concept
- Accounting Period and Matching Concept
- Cost Concept
- Realization Concept
- Substance over Form Concept
- Money measurement Concept
- Dual Aspect Concept
- Conservatism Convention
- Full Disclosure Convention
- Consistency Convention
- Materiality Convention
- Relevance and Reliability
- Comparability and Understandability
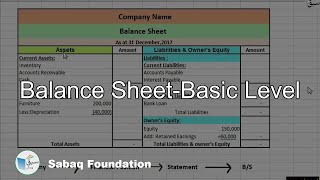
- Balance Sheet-Basic Level
- Asset
- Types of Assets
- Liabilities
- Equity
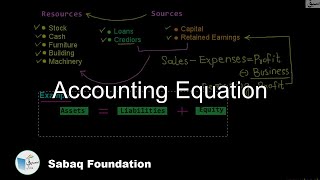
- Accounting Equation
- Accounting Equation (Problem)
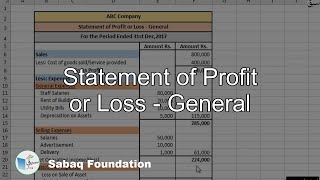
- Statement of Profit or Loss - General
Chapter 2 Basic Financial Statements ( 24 videos) (Practice Test)
2.1: Accounting Principles (Practice Test)

1466 views
2.2: Accounting Concepts (Practice Test)

1799 views

1367 views

1222 views

3558 views

4439 views

820 views

1871 views
2.3: Accounting Conventions (Practice Test)

675 views

1432 views

602 views

604 views
2.4: Qualitative Characteristics of Financial Accounting Information (Practice Test)

1391 views

726 views
2.5: Balance Sheet-Basic Level
911 views

86 views

3497 views

61 views

51 views
2.6: Accounting Equation
760 views

812 views
2.7: Income Statement
589 views
2.8: Cash Flow Statement (Practice Test)






