Acids, Bases and Salts
Select a chapter above and press 'Show Content'. Click a video topic below to view.
- Introduction to Acids and Bases
- Concept of Acids
- Concept of Bases
- Arrhenius concept of Acids
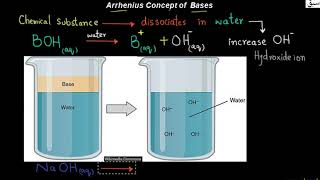
- Arrhenius Concept of Bases
- Limitations of Arrhenius Concept
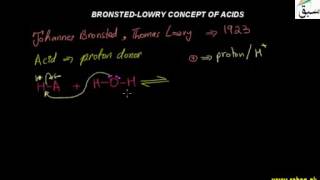
- Bronsted-Lowry Concept of Acids
- Bronsted-Lowry Concept of Bases
- Limitations of Bronsted-Lowry Concept
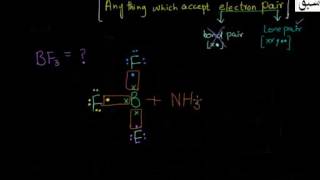
- Lewis concept of Acids
- Lewis concept of Bases
- Identifying Weak or Strong Acids or Bases
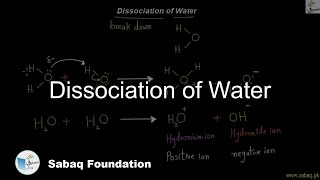
- Dissociation of Water
- The Ion Product of Water
- More on Ion Product of Water
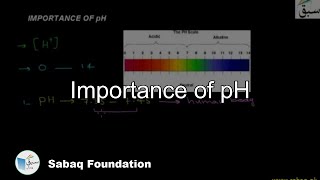
- Importance of pH
- Measuring of pH by Universal Indicator
- Indicators
- Calculating pH
- Calculating pOH
- Concept of Salts
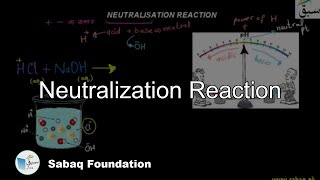
- Neutralization Reaction
- More on Neutralisation
- Types of Salts, Normal or Neutral Salts
- Acidic Salts
- Basic Salts
- Reaction of Bases with Acids
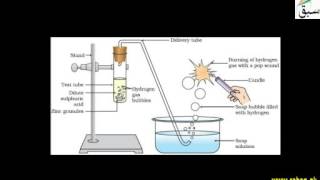
- Reaction of Acids with Metals
- Double Displacement Reactions
- Uses of Salts
Chapter 10 Acids, Bases and Salts ( 34 videos) (Practice Test)
10.1: Concepts of Acids and Bases (Practice Test)

3960 views

9746 views

5662 views
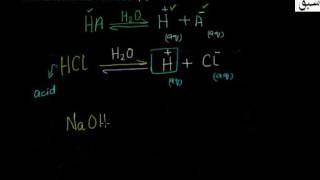
7507 views
4283 views

6501 views
11272 views

6976 views

4736 views
10425 views

5740 views

4699 views
10.2: Self-Ionization of Water -The pH Scale (Practice Test)
4709 views

6627 views

1322 views
346 views

2811 views

3980 views

5262 views

2983 views
10.3: Salts (Practice Test)

4385 views
1552 views

374 views

4899 views

3653 views

3106 views

2117 views
6379 views

1311 views
10.4: Uses of Salts

3798 views








