Support And Movement
Select a chapter above and press 'Show Content'. Click a video topic below to view.
- Genetic Causes
- Hormonal Causes of Skeleton Deformities
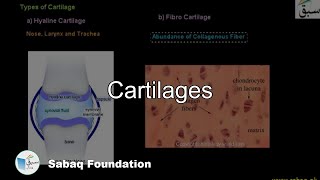
- Cartilages
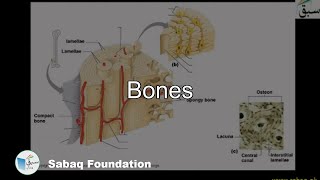
- Bones
- Human Skeleton, Axial Skeleton
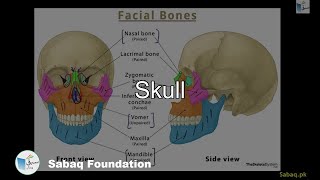
- Skull
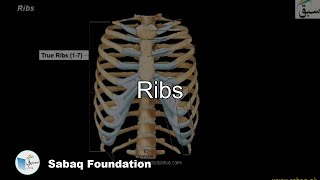
- Ribs
- Vertebral Column
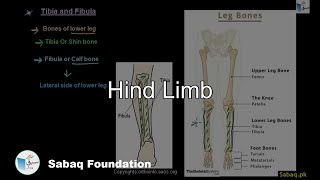
- Appendicular Skeleton
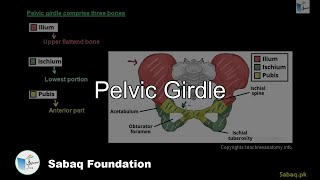
- Pelvic Girdle
- Types of Joints
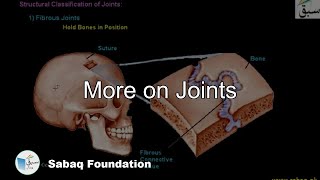
- More on Joints
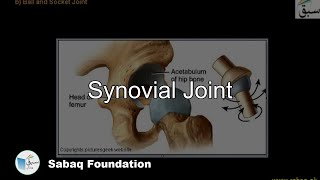
- Synovial Joint
- Disc-Slip
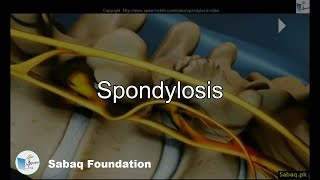
- Spondylosis
- Sciatica
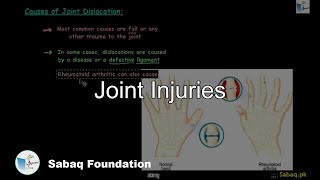
- Arthritis and Its Types
- Repair of Broken Bones
- Muscular System
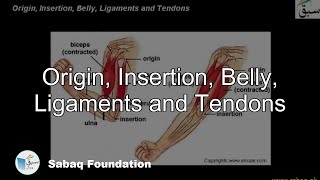
- Muscles and Movements
- Smooth and Cardiac Muscles
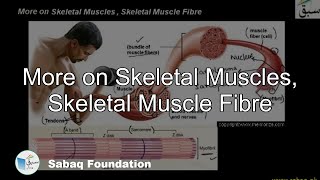
- Skeletal Muscles, Skeletal Muscle Fibre
- Ultrastructure of Myofilament
- Sliding Filament Model
- How Bridges are Controlled?
- Energy for Muscle Contraction
- Tetany and Cramp
Chapter 16 Support And Movement ( 33 videos) (Practice Test)
16.1: Human Skeleton (Practice Test)

3619 views

3743 views
6653 views
10019 views

38474 views
3485 views
2583 views

9 views

3189 views
3262 views

11695 views
3205 views
4692 views
16.2: Disorders of Skeleton (Practice Test)

6459 views
3761 views
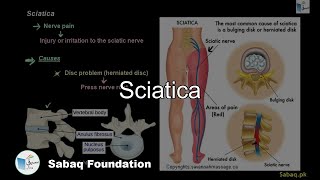
4201 views
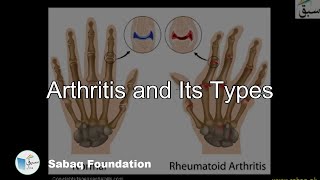
1679 views
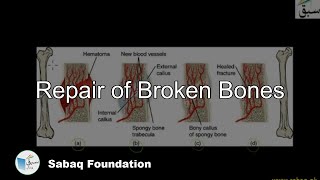
8478 views
16.3: Muscles (Practice Test)
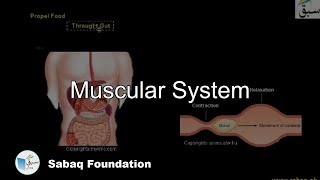
4186 views
7571 views

4437 views

12189 views
6912 views
8807 views
5968 views

6576 views
14328 views