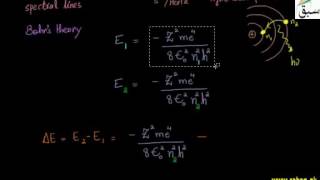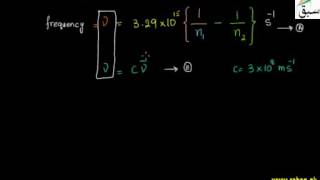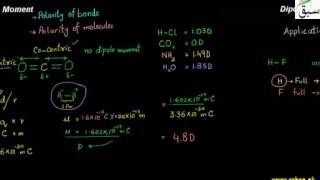Chemistry | Class 11
Select a chapter above and press 'Show Content'. Click a video topic below to view.
- Atom
- Evidence of Atoms
- Molecule
- Ions
- Molecular Ion
- More on Molecular Ion
- Relative Atomic Mass and Atomic Mass Unit
- Isotopes
- More on Isotopes
- Relative Abundance of Isotopes
- More on Determination of Ar of Isotopes
- Average Atomic Masses
- Calculating the Average Atomic Mass
- Calculate Percentage Abundance
- Calculating Percentage Composition
- Empirical Formula
- Empirical Formula from Combustion Analysis
- Molecular Formula
- More on Molecular Formula
- Determining Empirical Formula of a Compound
- Mole
- More on Concept of Mole
- Avogadro's Number
- More on Avogadro's Number
- Mole-Mass Calculations
- Mole-Mass Calculations
- Calculating Moles from Mass
- Calculating Mass from Moles
- Calculating Mass in Grams and Moles
- Mole-Particle Calculations
- More on Mole-Particle Calculations
- Calculating Mass in Grams of a Single Atom
- Calculating Number of Ions in the Compounds
- Calculating Number of Particles from Mass
- Molar Gas Volume
- Calculations with Molar Gas Volume
- Stoichiometry
- Stoichiometric Calculations
- Molar Calculations from Equations
- Limiting Reactants
- Importance of Limiting Reactants
- More on Importance of Limiting Reactants
- Calculating Limiting and Excess Reactants
Chapter 1 Basic Concepts ( 57 videos) (Practice Test)
1.1: Atom (Practice Test)

25566 views

11657 views
6657 views

4938 views
8846 views

3570 views
1.2: Relative Atomic Mass (Practice Test)

16670 views
1.3: Isotopes (Practice Test)

11479 views

2982 views

4339 views
2679 views

4441 views

6716 views
2649 views
1.4: Analysis of a Compound - Empirical and Molecular Formulas (Practice Test)

3356 views

13953 views

6951 views

11052 views
3352 views

1854 views
1.5: Concept of Mole (Practice Test)

32346 views

12151 views

18020 views

6942 views

5250 views

10808 views

11089 views

7590 views
7583 views

6535 views

3514 views

6457 views

6764 views

4824 views

5007 views

4359 views
1.6: Stoichiometry (Practice Test)

11001 views

2671 views

66 views
1.7: Limiting Reactant (Practice Test)

9105 views

4948 views

3816 views
5715 views
1.8: Yield (Practice Test)
- Filtration Through Filter Paper
- Filtration Through Filter Crucibles
- Choice of a Solvent
- Steps Involved in Crystallization
- More on Steps Involved in Crystallization
- Separating Solids Using Sublimation
- Solvent Extraction
- Distribution Law
- Chromatography
- Paper Chromatography
- More on Paper Chromatography
- Chromatography for Colourless Substances
- Uses of Chromatography
Chapter 2 Experimental Techniques in Chemistry ( 15 videos) (Practice Test)
2.1: Filtration (Practice Test)

5750 views

5131 views
2.2: Crystallization (Practice Test)

3069 views

3683 views

1998 views
2.3: Sublimation (Practice Test)

1942 views
2.4: Solvent Extraction (Practice Test)

17074 views

10692 views
2.5: Chromatography (Practice Test)
15599 views

7809 views

1957 views

1454 views

2855 views
- Shape and Volume of Gases
- Diffusion and Effusion in Gases
- Condensation
- Shape and Volume of Liquids
- Compressibility and Ease of Flow of Liquids
- Evaporation
- Dissolving, Filtering and Evaporating
- Shape and Volume of Solids
- Compressibility and Ease of Flow of Solids
- Melting
- Rigidity and Melting Point of Solids
- Density in Solids
- Diffusion in Solids
- Pressure and Standard Atmospheric Pressure
- Units of Pressure
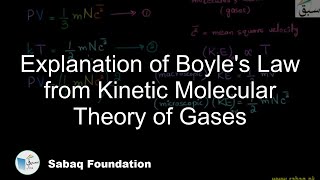
- Boyle's Law of Gases
- Experimental Verification of Boyle's Law
- Graphical Explanation of Boyle's Law
- Charles's Law
- Experimental Verification of Charles' Law
- Derivation of Absolute Zero
- Graphical Explanation of Charles' Law
- Measurement of Temperature
- Avogadro's Law
- Diffusion and Effusion in Gases
- Demonstration of Graham's Law
- Kinetic Molecular Theory of Gases
- More on Kinetic Molecular Theory of Gases
- Kinetic Interpretation of Temperature
- General Principle of Liquefaction
- Joule Thomson Effect
- Linde's Method of Liquefaction of Gases
- Non-Ideal Behaviour of Gases
- Causes for Deviations from Ideality
- Pressure Correction
- Proving Correction of Volume and Pressure
- Van der Waal's Constants
- Plasma
Chapter 3 Gases ( 53 videos) (Practice Test)
3.1: States of Matter (Practice Test)

5044 views

14761 views

4028 views

3996 views

3178 views

4143 views

1980 views

2576 views

3008 views

1769 views

3147 views
2487 views

2142 views

10827 views

8635 views
3.2: Gas Laws (Practice Test)
10840 views
9591 views
15727 views
11010 views

6915 views

19722 views
11236 views

2186 views
3.3: General Gas Equation (Practice Test)
3.4: Avogadro's Law (Practice Test)

9699 views
3.5: Dalton's Law of Partial Pressures (Practice Test)
3.6: Diffusion and Effusion (Practice Test)

14761 views

5747 views
3.7: Kinetic Molecular Theory of Gases (Practice Test)

13472 views
7628 views
3.8: Kinetic Interpretation of Temperature (Practice Test)

4489 views
3.9: Liquefaction of Gases (Practice Test)

5593 views

21153 views

29623 views
3.10: Non-Ideal Behaviour of Gases (Practice Test)

10622 views

7461 views

14510 views

5082 views

5087 views
3.11: Plasma State (Practice Test)
3727 views
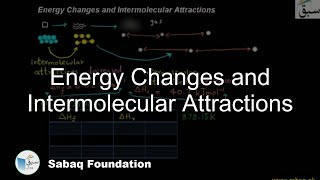
- Intermolecular Forces
- Dipole-Dipole Interactions
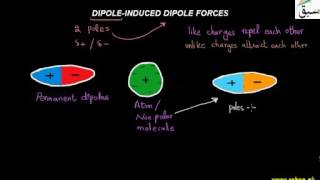
- Dipole-Induced Dipole Forces
- London Dispersion Forces
- Factors Affecting the London Forces
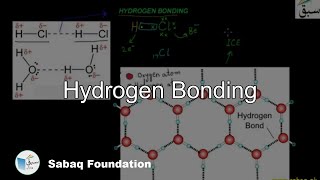
- Hydrogen Bonding
- Solubility of Hydrogen-Bonded Molecules
- Anomalous Behavior of Water
- More on Anomalous Behavior of Water
- Function of Soaps
- Function of Detergents
- Evaporation in Liquids and its Uses
- Evaporation
- Vapour Pressure of Liquids
- Effect of Temperature on Vapour Pressure
- Manometric Method of Measuring Vapour Pressure
- Boiling Points of Liquids
- Boiling Point and External Pressure
- Energetics of Phase Changes
- Dynamic Chemical Equilibrium
- Liquid Crystals
- Crystalline Solids
- Amorphous Solids
- Properties of Crystalline Solids
- Anisotropy
- Symmetry and Habit of a Crystal
- Isomorphism
- Polymorphism
- Concept of Allotropy
- Transition Temperature
- Crystal Lattice
- Unit Cell
- Crystals and Their Classification
- More on Crystals and Their Classification
- Properties of Ionic Solids
- More on Properties of Ionic Solids
- Structure of Sodium Chloride
- Lattice Energy
- Covalent Solids
- Properties of Covalent Crystals
- Diamond
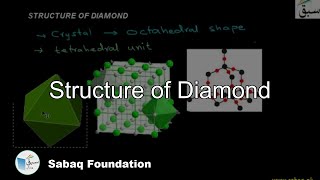
- Structure of Diamond
- Molecular Solids
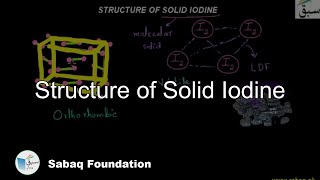
- Structure of Solid Iodine
- Metallic Solids
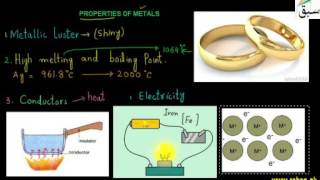
- Properties of Metals
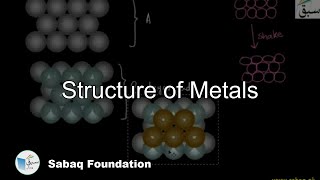
- Structure of Metals
- Determination of Avogadro's Number
Chapter 4 Liquids and Solids ( 52 videos) (Practice Test)
4.1: Intermolecular Forces (Practice Test)
13002 views
22680 views
16262 views

20887 views

8738 views
16302 views
3663 views

13729 views

3088 views

2519 views

1971 views
4.2: Evaporation (Practice Test)

7079 views

4143 views

8063 views
6069 views

13824 views

7380 views

3531 views

3112 views

17340 views
4.3: Liquid Crystals (Practice Test)

23584 views
4.4: Introduction to Solids (Practice Test)

9069 views

8167 views

11425 views
21074 views

8554 views

7381 views

8493 views

7697 views

4406 views
4.5: Crystal Lattice (Practice Test)

10127 views
5956 views
4.6: Crystals and Their Classification (Practice Test)

5911 views

1916 views
4.7: Classification of Solids (Practice Test)
4188 views

2174 views

19373 views
8422 views

4275 views

3205 views

4029 views
6318 views

5265 views
3878 views

6152 views
4009 views
5195 views
4.8: Determination of Avogadro's Number (NA) (Practice Test)

5270 views
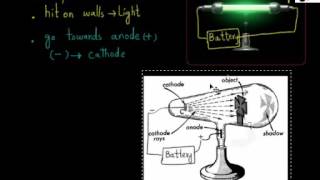
- Discovery of Electron (Cathode Rays)
- Properties of Cathode Rays
- More on Properties of Cathode rays
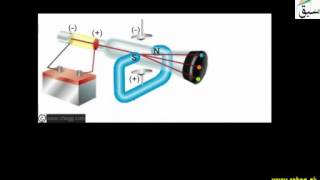
- Discovery of Proton
- Properties of Positive Rays
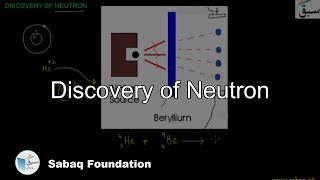
- Discovery of Neutron
- Properties of Neutron
- More on Properties of Neutron
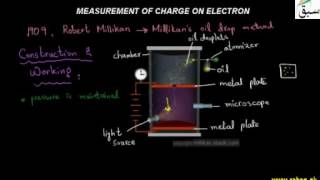
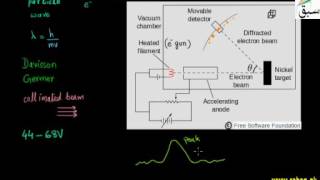
- Measurement of e/m Value of Electron
- More on Measurement of Charge on Electron
- Mass of Electron
- Properties of Fundamenal Particles
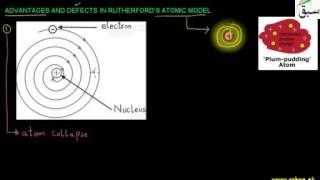
- Rutherford's Atomic Model
- Planck's Quantum Theory
- More on Planck's Quantum Theory
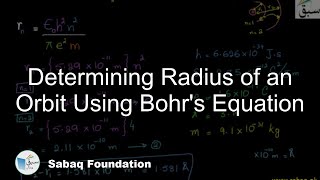
- Bohr's Atomic Theory
- Energy of Revolving Electron
- More on Energy of Revolving Electron
- Energy of Electron in nth Orbit
- Spectrum
- Continuous Spectrum
- Line Spectrum
- Atomic Emission Spectrum
- Atomic Absorption Spectrum
- Hydrogen Spectrum
- More on Hydrogen Spectrum
- Lyman Series
- Balmer Series
- Paschen Series
- Brackett series
- Pfund series
- Defects of Bohr's Atomic Model
- More on Defects of Bohr's Atomic Model
- X-Rays
- Types of X-rays
- Study of X-Rays by Moseley
- Importance of Moseley's Law
- Dual Nature of Matter
- More on Dual Nature of Matter
- Calculation of De-Broglie's Wavelength
- Heisenberg's Uncertainty Principle
- Concept of Orbital
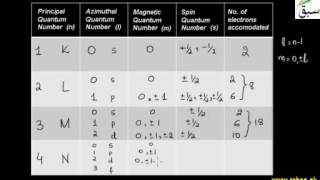
- Quantum Numbers
- Principal Quantum Numbers (n)
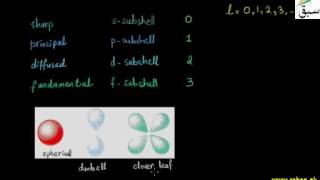
- Azimuthal Quantum Numbers (l)
- Magnetic Quantum Numbers (m)
- Spin Quantum Numbers (s)
- Quantum Numbers of Electrons
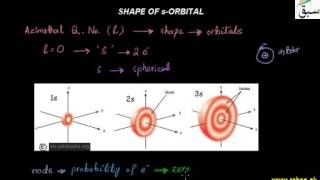
- Shape of s-Orbitals
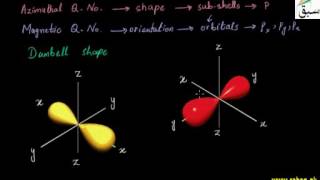
- Shapes of p-Orbitals
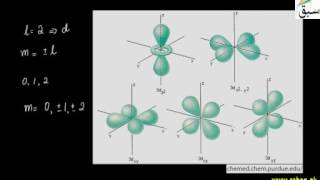
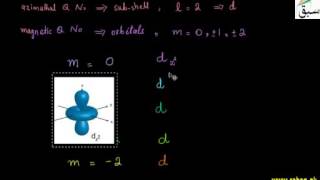
- Shapes of d-Orbitals
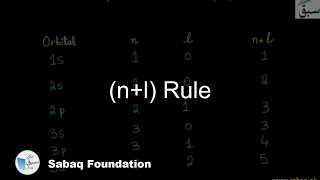
- (n+l) Rule
- Aufbau Principle
- Pauli's Exclusion Principle and Hund's Rule
- Electronic Configuration
- More on Electronic Configuration
Chapter 5 Atomic Structure ( 67 videos) (Practice Test)
5.1: Sub-Atomic Particles of Atom (Practice Test)
57392 views
19273 views
8537 views

76940 views

11665 views
26494 views
8357 views

3092 views

14362 views

7541 views
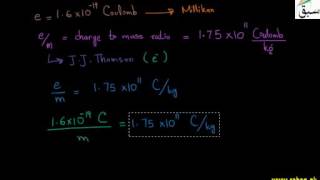
7839 views

1777 views
5.2: Rutherford's Model of Atom (Discovery of Nucleus) (Practice Test)

22654 views
5.3: Plank's Quantum Theory (Practice Test)

23564 views
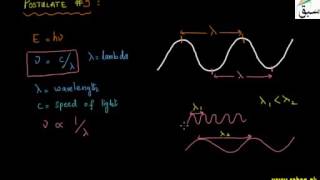
10436 views
5.4: Bohr's Model of Atom (Practice Test)
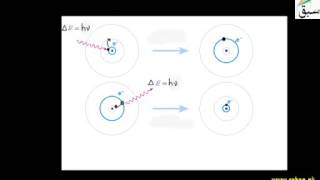
18709 views

12609 views

11208 views
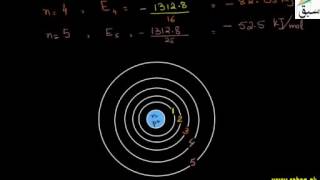
6675 views
5.5: Spectrum (Practice Test)
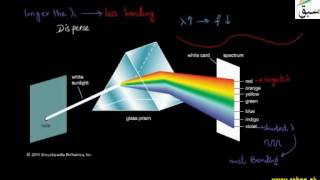
7220 views

5404 views
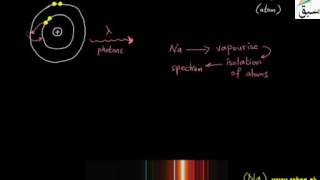
6859 views

6941 views

6420 views
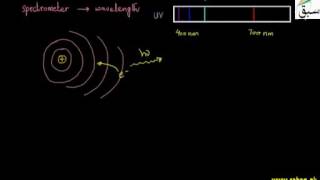
8402 views
7966 views
6565 views

3388 views

2831 views
2074 views

2006 views

11060 views

5793 views
5.6: X-Rays and Atomic Number (Practice Test)

8139 views
7569 views

7826 views
4281 views
5.7: Wave-Particle Nature of Matter (Dual Nature of Matter) (Practice Test)

6146 views
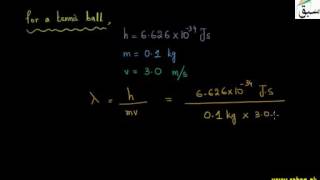
1475 views

2295 views
5.8: Heisenberg's Uncertainty Principle (Practice Test)

5002 views

9005 views

7543 views

6867 views
8684 views
17057 views

7239 views
3038 views
4220 views
8770 views
6002 views
5.9: Electronic Distribution (Practice Test)
10001 views

11901 views
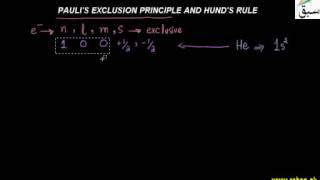
8672 views

13322 views

7168 views
- Why do Atoms Form Chemical Bonds?
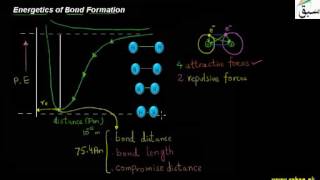
- Energetics of Bond Formation
- Atomic Size and Atomic Radius
- Trend of Atomic Radius in Periodic Table
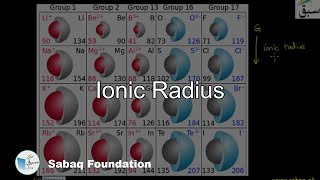
- Ionic Radius
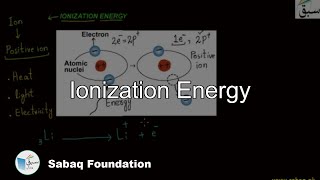
- Ionization Energy
- Factors Influencing the Ionization Energies
- Trend of Ionization Energy in Periodic Table
- Higher Ionization Energies
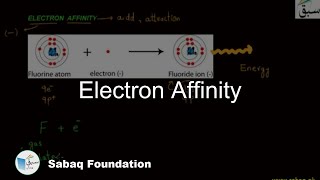
- Electron Affinity
- Factors Influencing the Electron Affinity
- Trend of Electron Affinity in Periodic Table
- More on Electronegativity
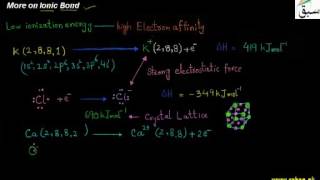
- Ionic Bond
- More on Ionic Bond
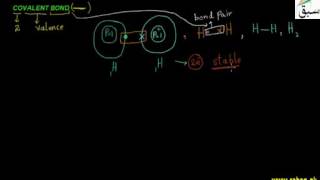
- Covalent Bond
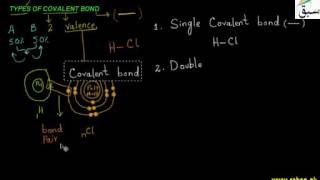
- Types of Covalent Bonds
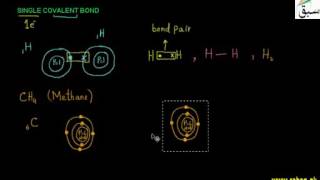
- Single Covalent Bond
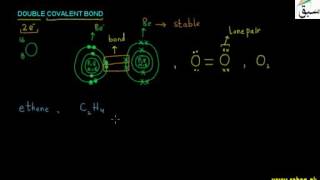
- Double Covalent Bond
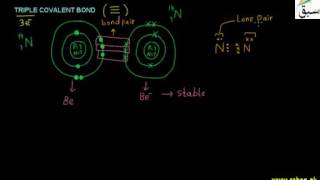
- Triple Covalent Bond
- Polar Covalent Bond
- Non polar Covalent Bond
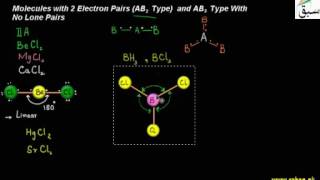
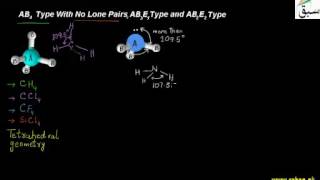
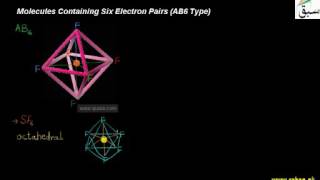
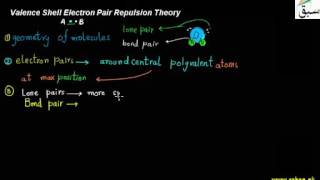
- Valence Shell Electron Pair Repulsion Theory
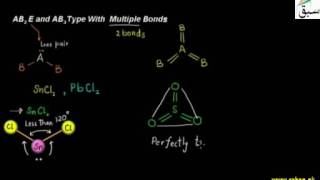
- AB2E and AB3 Type With Multiple Bonds
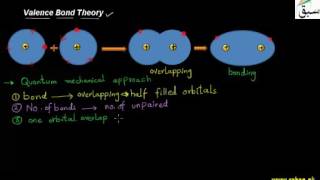
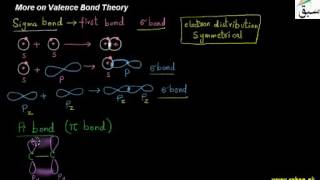
- Valence Bond Theory
- More on Valence Bond Theory
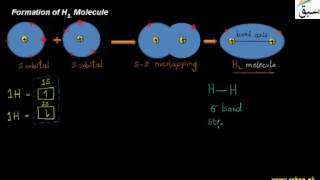
- Formation of H2 Molecule
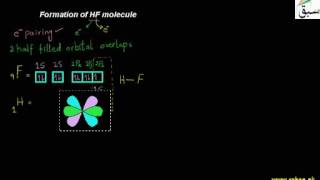
- Formation of HF molecule
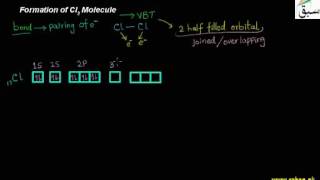
- Formation of Cl2 Molecule
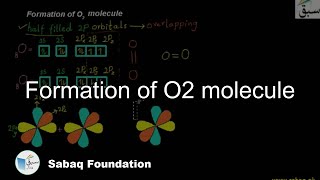
- Formation of O2 molecule
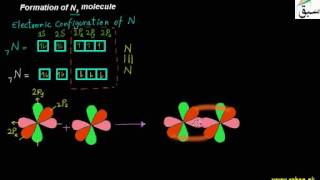
- Formation of N2 molecule
- Ground and Excited State of Carbon
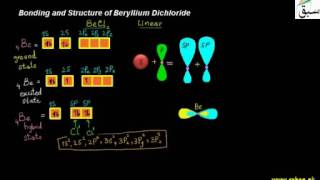
- Sp3 Hybridization
- More on Sp3 Hybridization
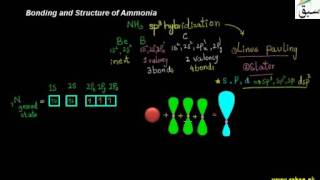
- Bonding and Structure of Ammonia
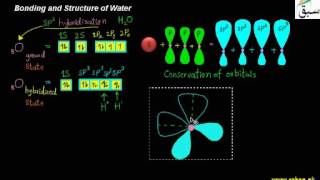
- Bonding and Structure of Water
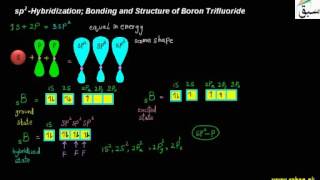
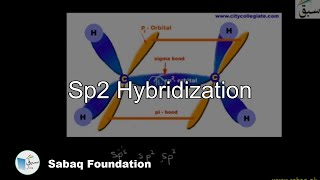
- Sp2 Hybridization
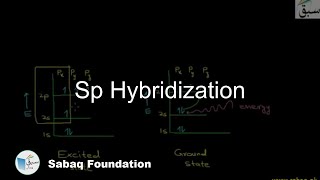
- Sp Hybridization
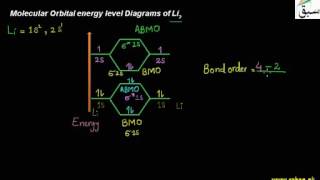
- Molecular Orbital Theory
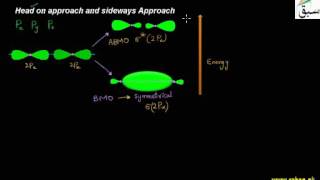
- Head on Approach and Sideways Approach
- Strength of Sigma and Pi Bonds
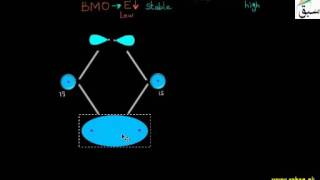
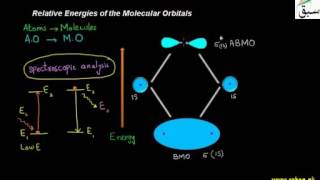
- Relative Energies of the Molecular Orbitals
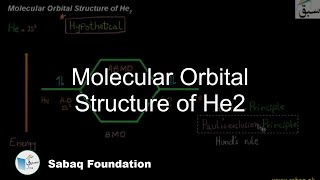
- Molecular Orbital Structure of He2
- MOT Diagrams for Be2, B2 and N2
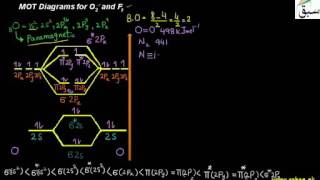
- MOT Diagrams for O2 and F2
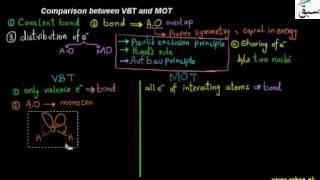
- Comparison between VBT and MOT
- Bond Energy
- Ionic Character and Bond Energy
- More on Ionic Character and Bond Energy
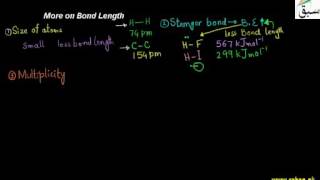
- Bond Length
- More on Bond Length
- Dipole Moment
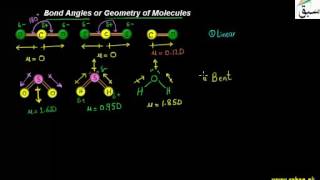
- Bond Angles or Geometry of Molecules
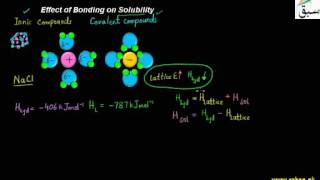
- Effect of Bonding on Solubility
- Effect of Bonding on Isomerism
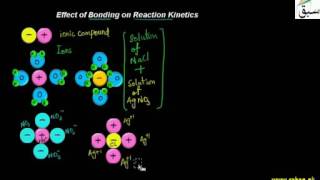
- Effect of Bonding on Reaction Kinetics
Chapter 6 Chemical Bonding ( 67 videos) (Practice Test)
6.1: Chemical Bond (Practice Test)

14190 views
6665 views
6.2: Atomic Sizes (Practice Test)

14240 views

27707 views
7458 views
6.3: Ionization Energy, Electron Affinity and Electronegativity (Practice Test)
10144 views

3292 views

14213 views

1190 views
8741 views

1523 views

5145 views

1061 views
6.4: Types of Bonds (Practice Test)
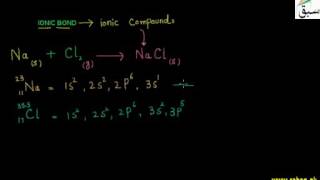
12605 views
2401 views
9028 views
6542 views
5587 views
4669 views
4585 views

7010 views

5689 views
15775 views
6796 views
25465 views
8712 views
5058 views
2097 views
3005 views
6875 views
5704 views

10197 views

10994 views

5637 views
7664 views
4856 views
8929 views
7843 views
21482 views
5529 views

6454 views
7218 views
4518 views

8732 views
15691 views
5225 views
6.5: Bond Energy, Bond Length and Dipole Moment (Practice Test)

6821 views

5793 views

3070 views

5462 views
2438 views

9837 views
1963 views
6.6: The Effect of Bonding on the Properties of Compounds (Practice Test)
1691 views

1246 views
1343 views
- Introduction to Thermochemistry
- Spontaneous and Non-Spontaneous Reactions
- work and Heat
- First Law of Thermodynamics
- Enthalpy
- Enthalpy Change of Different Reactions
- Enthalpy of Formation
- Enthalpy of Atomization
- Enthalpy of Neutralization
- Enthalpy of Combustion
- Calorimetry
- Hess's Law of Constant Heat Summation
- Verification of Hess's Law
- The Born-Haber Cycle
Chapter 7 Thermochemistry ( 19 videos) (Practice Test)
7.1: Spontaneous and Non-Spontaneous Reactions (Practice Test)
6121 views

5322 views
7.2: System, Surrounding and State Function (Practice Test)
7.3: Internal Energy and First Law of Thermodynamics (Practice Test)

6239 views
8951 views
7.4: Enthalpy (Practice Test)
7839 views

4494 views

6382 views
5634 views
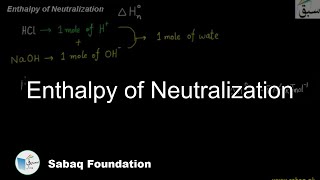
3368 views

2699 views
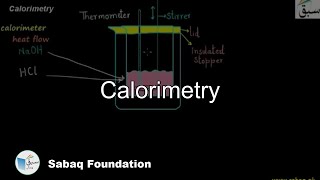
7515 views
7.5: Hess's Law of Constant Heat Summation (Practice Test)

10709 views

6295 views

13811 views
- Irreversible Chemical Reactions
- Reversible Chemical Reactions
- Static Chemical Equilibrium
- Dynamic Chemical Equilibrium
- Concept of Law of Mass Action
- Derivation of Law of Mass Action
- More on Derivation of Law of Mass Action
- Application of Law of Mass Action
- Equilibrium Constant Kc and its Units
- More on Units of Equilibrium Constant
- Relationship Between Equilibrium Constants
- Extent of Reaction
- Effect of Change in Pressure or Volume
- Effect of Change in Temperature
- Effect of Catalyst on Equilibrium Constant
- Preparation of Ammonia in Industry
- More on Haber process
- Preparation of Sulphur Trioxide
- Dissociation of Water
- The Ion Product of Water
- More on Ion Product of Water
- pH, pOH and pKw
- Ionization Constants of Acids
- Percentage of Ionization of Acids
- Ostwald's Dilution Law
- Ionization Constant of Bases
- pKa and pKb
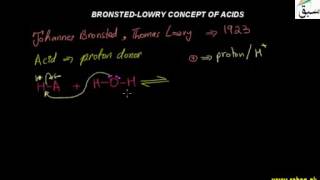
- Bronsted-Lowry Concept of Acids
- Bronsted-Lowry Concept of Bases
- Relationship between Ka and Kb
- Common Ion Effect
- Some Examples of Common Ion Effect
- Buffer Solutions
- Buffer Action
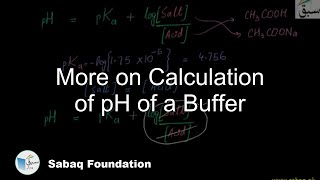
- Calculation of pH of a Buffer
- More on Calculation of pH of a Buffer
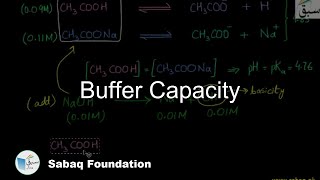
- Buffer Capacity
- Solubility Product
Chapter 8 Chemical Equilibrium ( 49 videos) (Practice Test)
8.1: Reversible and Irreversible Reactions (Practice Test)

4882 views

7787 views

19502 views

17340 views

18974 views

8344 views

5872 views

6468 views

12909 views

1352 views

3996 views

5837 views

6354 views

4117 views
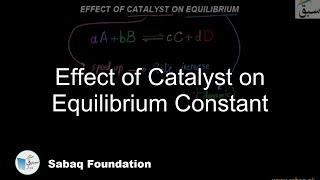
3126 views
8.2: Applications of Chemical Equilibrium in Industry (Practice Test)

5121 views

3069 views

2079 views
8.3: Ionic Product of Water (Practice Test)
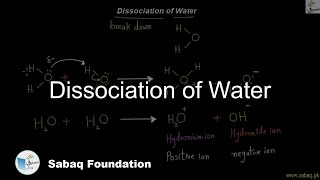
4709 views

6627 views

1322 views

8410 views
8.4: Ionization Constants of Acids (Ka) (Practice Test)

3519 views

1306 views

5314 views
8.5: Ionization Constant of Bases (Kb) (Practice Test)

1892 views

4131 views
8.6: Lowry Bronsted Acid and Base Concept (Practice Test)
11272 views

6976 views

3967 views
8.7: Common Ion Effect (Practice Test)

6805 views

4600 views
8.8: Buffer Solutions (Practice Test)

8461 views

12594 views

3714 views
2232 views
4214 views
8.9: Equilibria of Slightly Soluble Ionic Compounds (Solubility Product) (Practice Test)

7938 views
- Introduction to Solutions
- Concentration Units
- Percentage Mass/Mass
- Percentage Mass/Volume
- Percentage Volume/Mass
- Percentage Volume/Volume
- Moles and Solution
- Molarity and Preparation of Molar Solution
- Molality
- Mole Fraction (x)
- Solutions of Solids in Liquids
- Partially Miscible Liquids
- Phenol-Water System
- Ideal and Non-Ideal Solutions
- Raoult's Law
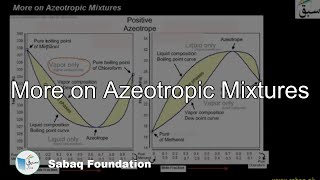
- Azeotropic Mixtures
- More on Azeotropic Mixtures
- Positive Deviations
- Negative Deviations
- Introduction to Solubility
- Solubility and Solute-Solvent Interactions
- Solubility Curves
- Fractional Crystallization
- Colligative Properties
- More on Colligative Properties
- Lowering of Vapour Pressure
- Elevation of Boiling Point
- More on Elevation of Boiling Point
- Measurement of Freezing Point Depression
- Energetics of Solution
- Hydration Energy of Ions
- Hydration
- Water of Crystallization
- Hydrolysis
- More on Hydrolysis
Chapter 9 Solutions ( 46 videos) (Practice Test)
9.1: Concentration Units of Solutions (Practice Test)

9101 views

4906 views
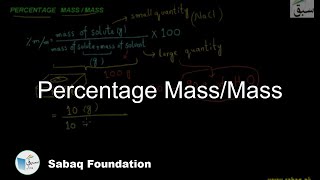
6518 views
5106 views

3978 views

3822 views

1314 views

7927 views
4009 views
2095 views
9.2: Types of Solutions (Practice Test)

2876 views

4612 views

15162 views
9.3: Ideal and Non-Ideal Solutions (Practice Test)

3735 views
13616 views
9.4: Vapour Pressures of Liquid-Liquid Solutions (Practice Test)

9938 views
5374 views

2195 views

1299 views
9.5: Solubility and Solubility Curves (Practice Test)
8696 views
8133 views

3268 views
2737 views
9.6: Colligative Properties of Solutions (Practice Test)

5682 views

2265 views

8224 views

10490 views

2034 views

7192 views
9.7: Energetics of Solution (Practice Test)

4472 views
5811 views
9.8: Hydration and Hydrolysis (Practice Test)
5212 views

4679 views

6664 views

3418 views
- Introduction to Electrochemistry
- Oxidation State
- More on Oxidation State
- Finding out the Oxidation Numbers
- Introduction to Electrolysis
- Explaining Electrolysis
- Electrolytic cells
- Construction of an Electrolytic cell
- Working of Electrolytic cell
- Products of Electrolysis
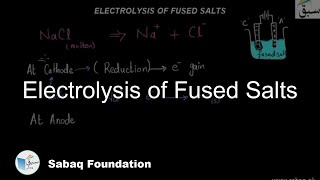
- Electrolysis of Fused Salts
- Electrolysis of Aqueous Solution of Salts
- Industrial Applications of Electrolysis
- Uses of Electrolysis
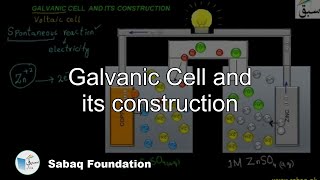
- Galvanic Cell and its construction
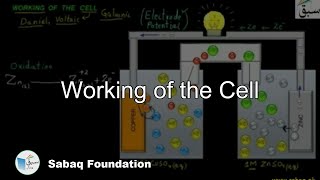
- Working of the Cell
- Voltaic Cell is Reversible Cell
- Electrode Potential
- Standard Hydrogen Electrode
- Measurement of Electrode Potential
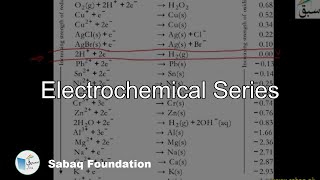
- Electrochemical Series
- Calculation of the Voltage or emf of Cells
- Relative Chemical Reactivity of Metals
- Reaction of Metals with Dilute Acids
- Lead-Storage Battery
- Discharging of Lead Battery
- Charging of Lead Battery
- Alkaline Battery
- Silver Oxide Battery
- Nickel Cadmium Cell
- Fuel Cells
- More on Fuel Cells
Chapter 10 Electrochemistry ( 43 videos) (Practice Test)
10.1: Oxidation State and Balancing of Redox Equations (Practice Test)

14155 views
5016 views

1599 views

9889 views
10.2: Electrolytic Conduction (Practice Test)
4837 views

2352 views

7932 views

3889 views

4206 views

3122 views
2165 views

4289 views

2604 views

3706 views
16981 views
5637 views

4768 views
10.3: Electrode Potential (Practice Test)

12052 views

17548 views

12025 views
10.4: The Electrochemical Series (Practice Test)
7619 views

1810 views
962 views

1595 views
10.5: Modern Batteries and Fuel Cells (Practice Test)

7002 views

8349 views

4668 views

9664 views

4624 views

11927 views

5549 views

1495 views
- Introduction to Reaction Kinetics
- Rate of Chemical Change
- Instantaneous and Average Rate
- Specific Rate Constant or Velocity Constant
- Order of Reaction
- Half Life Period
- Rate Determining Step
- Chemical Method
- Activation Energy
- More on Activation Energy
- Finding the Order of Reaction
- Effect of Concentration on Speed of Reaction
- Effect of Particle Size on Speed of Reaction
- Effect of Light on Speed of Reaction
- Effect of Temperature on Speed of Reaction
- Arrhenius Equation
Chapter 11 Reaction Kinetics ( 30 videos) (Practice Test)
11.1: Rate of Reaction (Practice Test)

7241 views

6074 views

6059 views

6995 views

11080 views

7820 views

5767 views
11.2: Determination of the Rate of a Chemical Reaction (Practice Test)

2754 views
11.3: Energy of Activation (Practice Test)

7413 views

3962 views
11.4: Finding the Order of Reaction (Practice Test)

3548 views
11.5: Factors Affecting Rates Of Reactions (Practice Test)

3542 views

1908 views

1460 views

3202 views

11317 views
11.6: Catalysis (Practice Test)