Solutions and Colloids
Select a chapter above and press 'Show Content'. Click a video topic below to view.
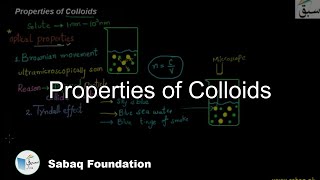
- Introduction to Solutions
- Colloids
- Suspensions
- Hydrophillic and Hydrophobic Molecules
- The Nature of Solution in Liquid Phase
- Partially Miscible Liquids
- Phenol-Water System
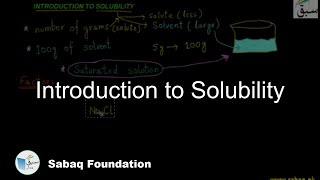
- Introduction to Solubility
- Solubility and Nature of Solute
- Solubility and Nature of Solvent
- The Effect of Pressure
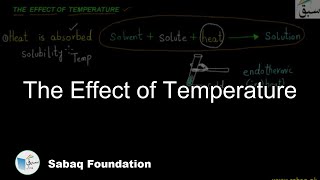
- The Effect of Temperature
- Solubility Curves
- Concentration Units
- Percentage Mass/Mass
- Percentage Mass/Volume
- Percentage Volume/Mass
- Percentage Volume/Volume
- Moles and Solution
- Molarity and Preparation of Molar Solution
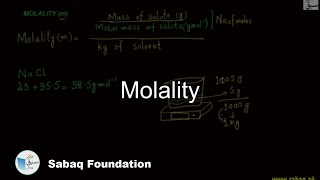
- Molality
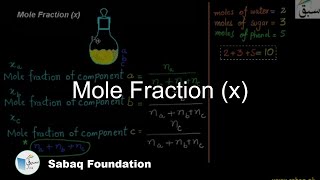
- Mole Fraction (x)
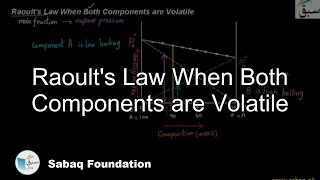
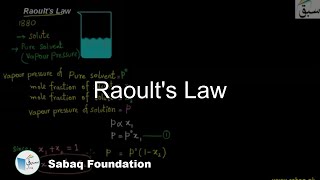
- Raoult's Law
- Colligative Properties
- More on Colligative Properties
- Lowering of Vapour Pressure
- Elevation of Boiling Point
- More on Elevation of Boiling Point
- Measurement of Boiling Point Elevation
- Osmosis and Osmotic Pressure
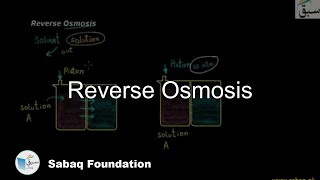
- Reverse Osmosis
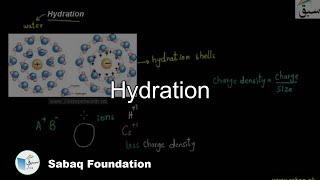
- Hydration
- Hydration Energy of Ions
Chapter 10 Solutions and Colloids ( 43 videos) (Practice Test)
10.1: General Properties of Solutions (Practice Test)

9101 views

2997 views

1073 views

4871 views

1730 views

4612 views

15162 views
8696 views

2618 views

2006 views

1702 views
5003 views

3268 views
10.2: Concentration Units (Practice Test)

4906 views
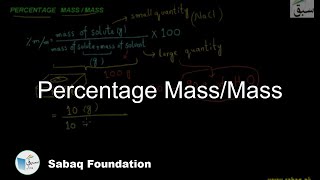
6518 views
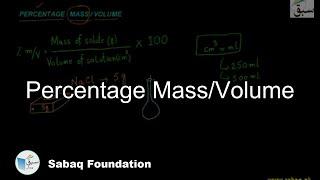
5106 views

3978 views

3822 views

1314 views

7927 views
4009 views
2095 views
10.3: Raoult's Law (Practice Test)
13616 views
10.4: Colligative Properties of Dilute Solutions (Practice Test)

5682 views

2265 views

8224 views

10490 views

2034 views

5606 views

9115 views
1801 views
5212 views
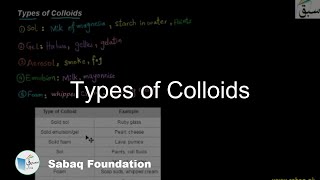
10.5: Classification of Colloids (Practice Test)
10.6: Heat of Solution and Its Application
5811 views