Inheritance
Select a chapter above and press 'Show Content'. Click a video topic below to view.
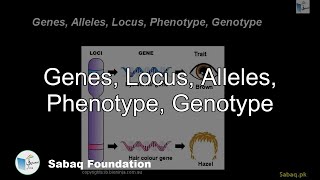
- Genes, Locus, Alleles, Phenotype, Genotype
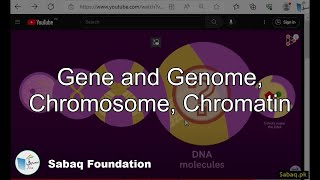
- Gene and Genome, Chromosome, Chromatin
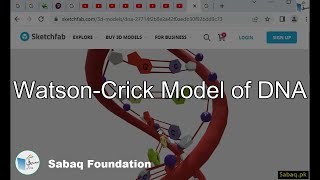
- Watson-Crick Model of DNA
- How Does the DNA of Chromosome work?
- Replication of DNA
- Transcription
- Translation
- Mendel’s Law of Segregation
- Mendel’s Law of Independent Assortment
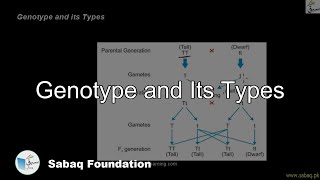
- Punnet Square, Test Cross
- Codominance
- Incomplete Dominance
- Sources of Variations
- Discontinuous and Continuous Variations
- Variations lead to Evolution
- Theory of Natural Selection
- Artificial Selection
Chapter 15 Inheritance ( 18 videos) (Practice Test)
15.1: Introduction to Genetics (Practice Test)
22120 views
15.2: Chromosomes and Genes (Practice Test)
11553 views
65841 views

7297 views
16059 views
18325 views
15338 views
15.3: Mendel’s Law of Inheritance (Practice Test)
28037 views

19410 views
4894 views
15.4: Co-Dominance and Incomplete Dominance (Practice Test)

8185 views

8390 views
15.5: Variations and Evolution (Practice Test)
3749 views

9470 views

5196 views

11343 views

9753 views